(1) Kim, J.; Kim, J.; Shim, J.; Lee, C. Y.; Lee, K. W.; Lee, H. J. Cocoa Phytochemicals: Recent Advances in Molecular Mechanisms on Health. Crit. Rev. Food Sci. Nutr.2014, 54 (11), 1458–1472. https://doi.org/10.1080/10408398.2011.641041.
(2) McShea, A.; Ramiro-Puig, E.; Munro, S. B.; Casadesus, G.; Castell, M.; Smith, M. A. Clinical Benefit and Preservation of Flavonols in Dark Chocolate Manufacturing. Nutr. Rev.2008, 66 (11), 630–641. https://doi.org/10.1111/j.1753-4887.2008.00114.x.
(3) Fraga, C. G.; Litterio, M. C.; Prince, P. D.; Calabró, V.; Piotrkowski, B.; Galleano, M. Cocoa Flavanols: Effects on Vascular Nitric Oxide and Blood Pressure. J. Clin. Biochem. Nutr.2011, 48 (1), 63–67. https://doi.org/10.3164/jcbn.11-010FR.
(4) Goya, L.; Martín, M. Á.; Sarriá, B.; Ramos, S.; Mateos, R.; Bravo, L. Effect of Cocoa and Its Flavonoids on Biomarkers of Inflammation: Studies of Cell Culture, Animals and Humans. Nutrients2016, 8 (4). https://doi.org/10.3390/nu8040212.
(5) Keen, C. L.; Holt, R. R.; Oteiza, P. I.; Fraga, C. G.; Schmitz, H. H. Cocoa Antioxidants and Cardiovascular Health. Am. J. Clin. Nutr.2005, 81 (1), 298S-303S. https://doi.org/10.1093/ajcn/81.1.298S.
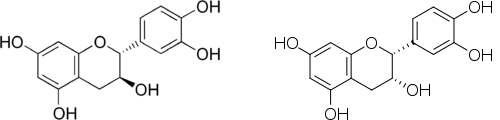
(6) Cádiz-Gurrea, M. L.; Lozano-Sanchez, J.; Contreras-Gámez, M.; Legeai-Mallet, L.; Fernández-Arroyo, S.; Segura-Carretero, A. Isolation, Comprehensive Characterization and Antioxidant Activities of Theobroma Cacao Extract. J. Funct. Foods2014, 10, 485–498. https://doi.org/10.1016/j.jff.2014.07.016.
(7) Davison, K.; Howe, P. R. C. Potential Implications of Dose and Diet for the Effects of Cocoa Flavanols on Cardiometabolic Function. J. Agric. Food Chem.2015, 63 (45), 9942–9947. https://doi.org/10.1021/acs.jafc.5b01492.
(8) Ellam, S.; Williamson, G. Cocoa and Human Health. Annu. Rev. Nutr.2013, 33 (1), 105–128. https://doi.org/10.1146/annurev-nutr-071811-150642.
(9) Murphy, K. J.; Chronopoulos, A. K.; Singh, I.; Francis, M. A.; Moriarty, H.; Pike, M. J.; Turner, A. H.; Mann, N. J.; Sinclair, A. J. Dietary Flavanols and Procyanidin Oligomers from Cocoa (Theobroma Cacao) Inhibit Platelet Function. Am. J. Clin. Nutr.2003, 77 (6), 1466–1473. https://doi.org/10.1093/ajcn/77.6.1466.
(10) Mastroiacovo, D.; Kwik-Uribe, C.; Grassi, D.; Necozione, S.; Raffaele, A.; Pistacchio, L.; Righetti, R.; Bocale, R.; Lechiara, M. C.; Marini, C.; Ferri, C.; Desideri, G. Cocoa Flavanol Consumption Improves Cognitive Function, Blood Pressure Control, and Metabolic Profile in Elderly Subjects: The Cocoa, Cognition, and Aging (CoCoA) Study—a Randomized Controlled Trial. Am. J. Clin. Nutr.2015, 101 (3), 538–548. https://doi.org/10.3945/ajcn.114.092189.
(11) Aging and vascular responses to flavanol-rich cocoa | Ovid https://oce-ovid-com.helicon.vuw.ac.nz/article/00004872-200608000-00017/HTML.
(12) Phaniendra, A.; Jestadi, D. B.; Periyasamy, L. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Indian J. Clin. Biochem. IJCB2015, 30 (1), 11–26. https://doi.org/10.1007/s12291-014-0446-0.
(13) Taubert, D.; Roesen, R.; Schömig, E. Effect of Cocoa and Tea Intake on Blood Pressure: A Meta-Analysis. Arch. Intern. Med.2007, 167 (7), 626–634. https://doi.org/10.1001/archinte.167.7.626.
(14) Ried, K.; Sullivan, T.; Fakler, P.; Frank, O. R.; Stocks, N. P. Does Chocolate Reduce Blood Pressure? A Meta-Analysis. BMC Med.2010, 8, 39. https://doi.org/10.1186/1741-7015-8-39.
(15) Wang, J. F.; Schramm, D. D.; Holt, R. R.; Ensunsa, J. L.; Fraga, C. G.; Schmitz, H. H.; Keen, C. L. A Dose-Response Effect from Chocolate Consumption on Plasma Epicatechin and Oxidative Damage. J. Nutr.2000, 130 (8), 2115S-2119S. https://doi.org/10.1093/jn/130.8.2115S.
(16) Singleton, V. L.; Orthofer, R.; Lamuela-Raventós, R. M. [14] Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Methods in Enzymology; Oxidants and Antioxidants Part A; Academic Press, 1999; Vol. 299, pp 152–178. https://doi.org/10.1016/S0076-6879(99)99017-1.
(17) Protective Effects of Flavanol-Rich Dark Chocolate on Endothelial Function and Wave Reflection During Acute Hyperglycemia | Hypertension https://www.ahajournals.org/doi/10.1161/HYPERTENSIONAHA.112.193995 (accessed May 23, 2021).
(18) Sansone, R.; Rodriguez-Mateos, A.; Heuel, J.; Falk, D.; Schuler, D.; Wagstaff, R.; Kuhnle, G. G. C.; Spencer, J. P. E.; Schroeter, H.; Merx, M. W.; Kelm, M.; Heiss, C.; for the Flaviola Consortium, E. U. 7th F. P. Cocoa Flavanol Intake Improves Endothelial Function and Framingham Risk Score in Healthy Men and Women: A Randomised, Controlled, Double-Masked Trial: The Flaviola Health Study. Br. J. Nutr.2015, 114 (8), 1246–1255. https://doi.org/10.1017/S0007114515002822.
(19) Bowe, W. P.; Patel, N.; Logan, A. C. Acne Vulgaris: The Role of Oxidative Stress and the Potential Therapeutic Value of Local and Systemic Antioxidants. J. Drugs Dermatol. JDD2012, 11 (6), 742–746.
(20) Al-Shobaili, H. A. Oxidants and Anti-Oxidants Status in Acne Vulgaris Patients with Varying Severity. Ann. Clin. Lab. Sci.2014, 44 (2), 202–207.
(21) Franco, R.; Oñatibia-Astibia, A.; Martínez-Pinilla, E. Health Benefits of Methylxanthines in Cacao and Chocolate. Nutrients2013, 5 (10), 4159–4173. http://dx.doi.org.helicon.vuw.ac.nz/10.3390/nu5104159.
(22) Martínez-Pinilla, E.; Oñatibia-Astibia, A.; Franco, R. The Relevance of Theobromine for the Beneficial Effects of Cocoa Consumption. Front. Pharmacol.2015, 6. https://doi.org/10.3389/fphar.2015.00030.
(23) Sugimoto, N.; Miwa, S.; Hitomi, Y.; Nakamura, H.; Tsuchiya, H.; Yachie, A. Theobromine, the Primary Methylxanthine Found in Theobroma Cacao, Prevents Malignant Glioblastoma Proliferation by Negatively Regulating Phosphodiesterase-4, Extracellular Signal-Regulated Kinase, Akt/Mammalian Target of Rapamycin Kinase, and Nuclear Factor-Kappa B. Nutr. Cancer2014, 66 (3), 419–423. https://doi.org/10.1080/01635581.2013.877497.
(24) Yoneda, M.; Sugimoto, N.; Katakura, M.; Matsuzaki, K.; Tanigami, H.; Yachie, A.; Ohno-Shosaku, T.; Shido, O. Theobromine Up-Regulates Cerebral Brain-Derived Neurotrophic Factor and Facilitates Motor Learning in Mice. J. Nutr. Biochem.2017, 39, 110–116. https://doi.org/10.1016/j.jnutbio.2016.10.002.
(25) Antioxidant and prooxidant properties of caffeine, theobromine and xanthine https://www.medscimonit.com/download/index/idArt/13137 (accessed May 30, 2021).
(26) Haskó György; Pacher Pál. Regulation of Macrophage Function by Adenosine. Arterioscler. Thromb. Vasc. Biol.2012, 32 (4), 865–869. https://doi.org/10.1161/ATVBAHA.111.226852.
(27) Barletta Kathryn E.; Ley Klaus; Mehrad Borna. Regulation of Neutrophil Function by Adenosine. Arterioscler. Thromb. Vasc. Biol.2012, 32 (4), 856–864. https://doi.org/10.1161/ATVBAHA.111.226845.
(28) Basheer, R.; Strecker, R. E.; Thakkar, M. M.; McCarley, R. W. Adenosine and Sleep–Wake Regulation. Prog. Neurobiol.2004, 73 (6), 379–396. https://doi.org/10.1016/j.pneurobio.2004.06.004.
(29) ZOUMAS, B.; KREISER, W.; MARTIN, R. Theobromine and Caffeine Content of Chocolate Products. J. Food Sci.2006, 45, 314–316. https://doi.org/10.1111/j.1365-2621.1980.tb02603.x.
(30) Mitchell, E. S.; Slettenaar, M.; vd Meer, N.; Transler, C.; Jans, L.; Quadt, F.; Berry, M. Differential Contributions of Theobromine and Caffeine on Mood, Psychomotor Performance and Blood Pressure. Physiol. Behav.2011, 104 (5), 816–822. https://doi.org/10.1016/j.physbeh.2011.07.027.
(31) Sharif, S.; Guirguis, A.; Fergus, S.; Schifano, F. The Use and Impact of Cognitive Enhancers among University Students: A Systematic Review. Brain Sci.2021, 11 (3), 355. https://doi.org/10.3390/brainsci11030355.
(32) Wee, J. H.; Min, C.; Park, M. W.; Park, I.-S.; Park, B.; Choi, H. G. Energy-Drink Consumption Is Associated with Asthma, Allergic Rhinitis, and Atopic Dermatitis in Korean Adolescents. Eur. J. Clin. Nutr.2020, 1–11. https://doi.org/10.1038/s41430-020-00812-2.
(33) Patocka, J.; Navratilova, Z.; Krejcar, O.; Kuca, K. Coffee, Caffeine and Cognition: A Benefit or Disadvantage? Lett. Drug Des. Discov.2019, 16 (10), 1146–1156. https://doi.org/10.2174/1570180816666190620142158.
(34) Lane, J. D.; Manus, D. C. Persistent Cardiovascular Effects with Repeated Caffeine Administration. Psychosom. Med.1989, 51 (4), 373–380.
(35) Francis, S. H.; Blount, M. A.; Corbin, J. D. Mammalian Cyclic Nucleotide Phosphodiesterases: Molecular Mechanisms and Physiological Functions. Physiol. Rev.2011, 91 (2), 651–690. https://doi.org/10.1152/physrev.00030.2010.
(36) van den Bogaard, B.; Draijer, R.; Westerhof, B. E.; van den Meiracker, A. H.; van Montfrans, G. A.; van den Born, B.-J. H. Effects on Peripheral and Central Blood Pressure of Cocoa with Natural or High-Dose Theobromine: A Randomized, Double-Blind Crossover Trial. Hypertens. Dallas Tex 19792010, 56 (5), 839–846. https://doi.org/10.1161/HYPERTENSIONAHA.110.158139.
(37) PubChem. Theobromine https://pubchem.ncbi.nlm.nih.gov/compound/5429 (accessed May 30, 2021).
(38) Gennaro, M. C.; Abrigo, C. Caffeine and Theobromine in Coffee, Tea and Cola-Beverages: Simultaneous Determination by Reversed-Phase Ion-Interaction HPLC. Fresenius J. Anal. Chem.1992, 343 (6), 523–525. https://doi.org/10.1007/BF00322162.
(39) Ikeda, M.; Tsuji, H.; Nakamura, S.; Ichiyama, A.; Nishizuka, Y.; Hayaishi, O. Studies on the Biosynthesis of Nicotinamide Adenine Dinucleotide. J. Biol. Chem.1965, 240 (3), 1395–1401. https://doi.org/10.1016/S0021-9258(18)97589-7.
(40) Wurtman, R. J.; Anton-tay, F. The Mammalian Pineal as a Neuroendocrine Transducer11Studies Described in This Report Were Supported by Grants from the National Aeronautics and Space Administration (NGR-22-009-272) and the National Institutes of Health (AM-11709 and AM-11237). In Proceedings of the 1968 Laurentian Hormone Conference; Astwood, E. B., Ed.; Recent Progress in Hormone Research; Academic Press: Boston, 1969; Vol. 25, pp 493–522. https://doi.org/10.1016/B978-0-12-571125-8.50014-4.
(41) Fernstrom, J. D. Role of Precursor Availability in Control of Monoamine Biosynthesis in Brain. Physiol. Rev.1983, 63 (2), 484–546. https://doi.org/10.1152/physrev.1983.63.2.484.
(42) Riedel, W. J.; Klaassen, T.; Schmitt, J. A. J. Tryptophan, Mood, and Cognitive Function. Brain. Behav. Immun.2002, 16 (5), 581–589. https://doi.org/10.1016/S0889-1591(02)00013-2.
(43) Silber, B. Y.; Schmitt, J. A. J. Effects of Tryptophan Loading on Human Cognition, Mood, and Sleep. Neurosci. Biobehav. Rev.2010, 34 (3), 387–407. https://doi.org/10.1016/j.neubiorev.2009.08.005.
(44) Jenkins, T. A.; Nguyen, J. C. D.; Polglaze, K. E.; Bertrand, P. P. Influence of Tryptophan and Serotonin on Mood and Cognition with a Possible Role of the Gut-Brain Axis. Nutrients2016, 8 (1). https://doi.org/10.3390/nu8010056.
(45) Aghajanian, G. K.; Marek, G. J. Serotonin, via 5-HT2A Receptors, Increases EPSCs in Layer V Pyramidal Cells of Prefrontal Cortex by an Asynchronous Mode of Glutamate Release. Brain Res.1999, 825 (1), 161–171. https://doi.org/10.1016/S0006-8993(99)01224-X.
(46) Marek, G. J.; Wright, R. A.; Gewirtz, J. C.; Schoepp, D. D. A Major Role for Thalamocortical Afferents in Serotonergic Hallucinogen Receptor Function in the Rat Neocortex. Neuroscience2001, 105 (2), 379–392. https://doi.org/10.1016/S0306-4522(01)00199-3.
(47) Bortolozzi, A.; Díaz‐Mataix, L.; Scorza, M. C.; Celada, P.; Artigas, F. The Activation of 5-HT2A Receptors in Prefrontal Cortex Enhances Dopaminergic Activity. J. Neurochem.2005, 95 (6), 1597–1607. https://doi.org/10.1111/j.1471-4159.2005.03485.x.
(48) Radulovacki, P.; Djuricic-Nedelson, M.; Chen, E. H.; Radulovacki, M. Human Tryptamine Metabolism Decreases during Night Sleep. Brain Res. Bull.1983, 10 (1), 43–45. https://doi.org/10.1016/0361-9230(83)90072-2.
(49) The Ayahuasca Phenomenon https://maps.org/articles/5408-the-ayahuasca-phenomenon (accessed May 16, 2021).
(50) Smith, R. L.; Canton, H.; Barrett, R. J.; Sanders-Bush, E. Agonist Properties of N,N-Dimethyltryptamine at Serotonin 5-HT2A and 5-HT2C Receptors. Pharmacol. Biochem. Behav.1998, 61 (3), 323–330. https://doi.org/10.1016/S0091-3057(98)00110-5.
(51) Daytime Ayahuasca administration modulates REM and slow-wave sleep in healthy volunteers | SpringerLink https://link-springer-com.helicon.vuw.ac.nz/article/10.1007/s00213-007-0963-0 (accessed May 30, 2021).
(52) Callaway, J. C. A Proposed Mechanism for the Visions of Dream Sleep. Med. Hypotheses1988, 26 (2), 119–124. https://doi.org/10.1016/0306-9877(88)90064-3.
(53) Wüst, N.; Rauscher-Gabernig, E.; Steinwider, J.; Bauer, F.; Paulsen, P. Risk Assessment of Dietary Exposure to Tryptamine for the Austrian Population. Food Addit. Contam. Part Chem. Anal. Control Expo. Risk Assess.2017, 34 (3), 404–420. https://doi.org/10.1080/19440049.2016.1269207.
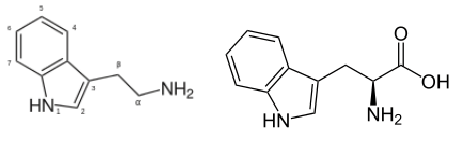
(54) PubChem. Phenethylamine https://pubchem.ncbi.nlm.nih.gov/compound/1001 (accessed May 17, 2021).
(55) Pei, Y.; Asif-Malik, A.; Canales, J. J. Trace Amines and the Trace Amine-Associated Receptor 1: Pharmacology, Neurochemistry, and Clinical Implications. Front. Neurosci.2016, 10. https://doi.org/10.3389/fnins.2016.00148.
(56) Miller, G. M. The Emerging Role of Trace Amine Associated Receptor 1 in the Functional Regulation of Monoamine Transporters and Dopaminergic Activity. J. Neurochem.2011, 116 (2), 164–176. https://doi.org/10.1111/j.1471-4159.2010.07109.x.
(57) Wimalasena, K. Vesicular Monoamine Transporters: Structure-Function, Pharmacology, and Medicinal Chemistry. Med. Res. Rev.2011, 31 (4), 483–519. https://doi.org/10.1002/med.20187.
(58) Xie, Z.; Miller, G. M. Beta-Phenylethylamine Alters Monoamine Transporter Function via Trace Amine-Associated Receptor 1: Implication for Modulatory Roles of Trace Amines in Brain. J. Pharmacol. Exp. Ther.2008, 325 (2), 617–628. https://doi.org/10.1124/jpet.107.134247.
(59) Grandy, D. K.; Miller, G. M.; Li, J.-X. “TAARgeting Addiction” The Alamo Bears Witness to Another Revolution. Drug Alcohol Depend.2016, 159, 9–16. https://doi.org/10.1016/j.drugalcdep.2015.11.014.
(60) Sotnikova, T. D.; Budygin, E. A.; Jones, S. R.; Dykstra, L. A.; Caron, M. G.; Gainetdinov, R. R. Dopamine Transporter-Dependent and -Independent Actions of Trace Amine Beta-Phenylethylamine. J. Neurochem.2004, 91 (2), 362–373. https://doi.org/10.1111/j.1471-4159.2004.02721.x.
(61) Barroso, N.; Rodriguez, M. Action of Beta-Phenylethylamine and Related Amines on Nigrostriatal Dopamine Neurotransmission. Eur. J. Pharmacol.1996, 297 (3), 195–203. https://doi.org/10.1016/0014-2999(95)00757-1.
(62) Ikemoto, S. Brain Reward Circuitry beyond the Mesolimbic Dopamine System: A Neurobiological Theory. Neurosci. Biobehav. Rev.2010, 35 (2), 129–150. https://doi.org/10.1016/j.neubiorev.2010.02.001.
(63) Irsfeld, M.; Spadafore, M.; Prüß, B. M. β-Phenylethylamine, a Small Molecule with a Large Impact. WebmedCentral2013, 4 (9).
(64) Sabelli, H.; Fink, P.; Fawcett, J.; Tom, C. Sustained Antidepressant Effect of PEA Replacement. J. Neuropsychiatry Clin. Neurosci.1996, 8 (2), 168–171. https://doi.org/10.1176/jnp.8.2.168.
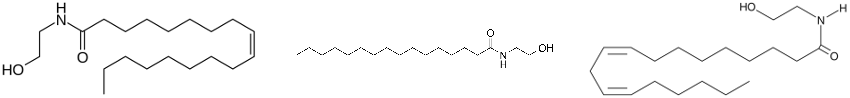
(65) Marzo, V. D.; Sepe, N.; Petrocellis, L. D.; Berger, A.; Crozier, G.; Fride, E.; Mechoulam, R. Trick or Treat from Food Endocannabinoids? Nature1998, 396 (6712), 636–636. https://doi.org/10.1038/25267.
(66) Tomaso, E. di; Beltramo, M.; Piomelli, D. Brain Cannabinoids in Chocolate. Nature1996, 382 (6593), 677–678. https://doi.org/10.1038/382677a0.
(67) Devane, W. A.; Hanus, L.; Breuer, A.; Pertwee, R. G.; Stevenson, L. A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and Structure of a Brain Constituent That Binds to the Cannabinoid Receptor. Science1992, 258 (5090), 1946–1949. https://doi.org/10.1126/science.1470919.
(68) Fuss, J.; Steinle, J.; Bindila, L.; Auer, M. K.; Kirchherr, H.; Lutz, B.; Gass, P. A Runner’s High Depends on Cannabinoid Receptors in Mice. Proc. Natl. Acad. Sci.2015, 112 (42), 13105–13108. https://doi.org/10.1073/pnas.1514996112.
(69) Hill, M. N.; Patel, S.; Campolongo, P.; Tasker, J. G.; Wotjak, C. T.; Bains, J. S. Functional Interactions between Stress and the Endocannabinoid System: From Synaptic Signaling to Behavioral Output. J. Neurosci.2010, 30 (45), 14980–14986. https://doi.org/10.1523/JNEUROSCI.4283-10.2010.
(70) Hwang, J.; Adamson, C.; Butler, D.; Janero, D. R.; Makriyannis, A.; Bahr, B. A. Enhancement of Endocannabinoid Signaling by Fatty Acid Amide Hydrolase Inhibition: A Neuroprotective Therapeutic Modality. Life Sci.2010, 86 (15–16), 615–623. https://doi.org/10.1016/j.lfs.2009.06.003.
(71) Gaetani, S.; Dipasquale, P.; Romano, A.; Righetti, L.; Cassano, T.; Piomelli, D.; Cuomo, V. Chapter 5 The Endocannabinoid System as A Target for Novel Anxiolytic and Antidepressant Drugs. In International Review of Neurobiology; Academic Press, 2009; Vol. 85, pp 57–72. https://doi.org/10.1016/S0074-7742(09)85005-8.
(72) Lambert, D. M.; Vandevoorde, S.; Diependaele, G.; Govaerts, S. J.; Robert, A. R. Anticonvulsant Activity of N-Palmitoylethanolamide, a Putative Endocannabinoid, in Mice. Epilepsia2001, 42 (3), 321–327. https://doi.org/10.1046/j.1528-1157.2001.41499.x.
(73) Calignano, A.; La Rana, G.; Piomelli, D. Antinociceptive Activity of the Endogenous Fatty Acid Amide, Palmitylethanolamide. Eur. J. Pharmacol.2001, 419 (2), 191–198. https://doi.org/10.1016/S0014-2999(01)00988-8.
(74) Verme, J. L.; Fu, J.; Astarita, G.; Rana, G. L.; Russo, R.; Calignano, A.; Piomelli, D. The Nuclear Receptor Peroxisome Proliferator-Activated Receptor-α Mediates the Anti-Inflammatory Actions of Palmitoylethanolamide. Mol. Pharmacol.2005, 67 (1), 15–19. https://doi.org/10.1124/mol.104.006353.
(75) Thabuis, C.; Tissot-Favre, D.; Bezelgues, J.-B.; Martin, J.-C.; Cruz-Hernandez, C.; Dionisi, F.; Destaillats, F. Biological Functions and Metabolism of Oleoylethanolamide. Lipids2008, 43 (10), 887. https://doi.org/10.1007/s11745-008-3217-y.
(76) Wiranda -; Syukur, S.; Aziz, H. DETERMINATION OF CALCIUM (Ca) AND MAGNESIUM (Mg) CONTENT IN CACAO (Theobroma Cacao Linn) FERMENTATION AND NON FERMENTATION BY SPECTROPHOTOMETRY. J. Ris. Kim.2015, 3 (1), 96. https://doi.org/10.25077/jrk.v3i1.104.
(77) Shittu, T. A.; Badmus, B. A. Statistical Correlations between Mineral Element Composition, Product Information and Retail Price of Powdered Cocoa Beverages in Nigeria. J. Food Compos. Anal.2009, 22 (3), 212–217. https://doi.org/10.1016/j.jfca.2008.10.006.
(78) Cherasse, Y.; Urade, Y. Dietary Zinc Acts as a Sleep Modulator. Int. J. Mol. Sci.2017, 18 (11). https://doi.org/10.3390/ijms18112334.
(79) Prakash, A.; Bharti, K.; Majeed, A. B. A. Zinc: Indications in Brain Disorders. Fundam. Clin. Pharmacol.2015, 29 (2), 131–149. https://doi.org/10.1111/fcp.12110.
(80) Rouault, T. A. How Mammals Acquire and Distribute Iron Needed for Oxygen-Based Metabolism. PLoS Biol.2003, 1 (3). https://doi.org/10.1371/journal.pbio.0000079.
(81) Iron https://lpi.oregonstate.edu/mic/minerals/iron (accessed May 17, 2021).
(82) Office of Dietary Supplements - Magnesium https://ods.od.nih.gov/factsheets/magnesium-HealthProfessional/ (accessed May 17, 2021).
(83) Long, S.; Romani, A. M. Role of Cellular Magnesium in Human Diseases. Austin J. Nutr. Food Sci.2014, 2 (10).
(84) de Baaij, J. H. F.; Hoenderop, J. G. J.; Bindels, R. J. M. Magnesium in Man: Implications for Health and Disease. Physiol. Rev.2015, 95 (1), 1–46. https://doi.org/10.1152/physrev.00012.2014.
(85) Calcium https://lpi.oregonstate.edu/mic/minerals/calcium (accessed May 17, 2021).
(86) Office of Dietary Supplements - Calcium https://ods.od.nih.gov/factsheets/Calcium-HealthProfessional/ (accessed May 17, 2021).
(87) Bailey, R. L.; Dodd, K. W.; Goldman, J. A.; Gahche, J. J.; Dwyer, J. T.; Moshfegh, A. J.; Sempos, C. T.; Picciano, M. F. Estimation of Total Usual Calcium and Vitamin D Intakes in the United States. J. Nutr.2010, 140 (4), 817–822. https://doi.org/10.3945/jn.109.118539.
(88) Clark, S. F. Iron Deficiency Anemia. Nutr. Clin. Pract. Off. Publ. Am. Soc. Parenter. Enter. Nutr.2008, 23 (2), 128–141. https://doi.org/10.1177/0884533608314536.
(89) WHO | Assessing the iron status of populations http://www.who.int/nutrition/publications/micronutrients/anaemia_iron_deficiency/9789241596107/en/ (accessed May 17, 2021).
(90) McLean, E.; Cogswell, M.; Egli, I.; Wojdyla, D.; de Benoist, B. Worldwide Prevalence of Anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993-2005. Public Health Nutr.2009, 12 (4), 444–454. https://doi.org/10.1017/S1368980008002401.
(91) Rosanoff, A.; Weaver, C. M.; Rude, R. K. Suboptimal Magnesium Status in the United States: Are the Health Consequences Underestimated? Nutr. Rev.2012, 70 (3), 153–164. https://doi.org/10.1111/j.1753-4887.2011.00465.x.
(92) Jamison JR. Mineral Deficiency: A Dietary Dilemma? J. Nutr. Environ. Med.1999, 9 (2), 149–158. https://doi.org/10.1080/13590849961744.
(93) Nutrient Reference Values for Australia and New Zealand https://www.health.govt.nz/publication/nutrient-reference-values-australia-and-new-zealand (accessed May 23, 2021).